PDF) Electron screening effect in the reactions 3He(d, p) 4He and d( 3He, p) 4He Supported in part by INFN, BMBF (06BO812), DFG (436UNG113-146) and OTKA (T025465 | Matthias Junker -

Color online) (a) Valence-electron screening cloud around the excited... | Download Scientific Diagram

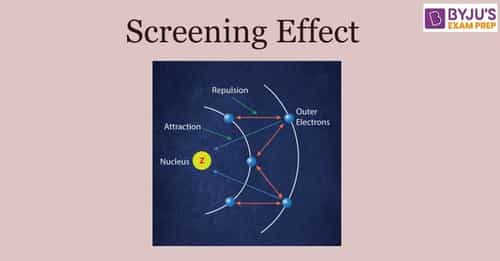
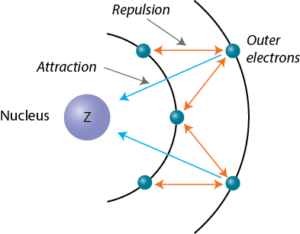
What are Slater's rules for calculating screening constant? | by Chemistry Topics | Inorganic Chemistry Topics | Medium

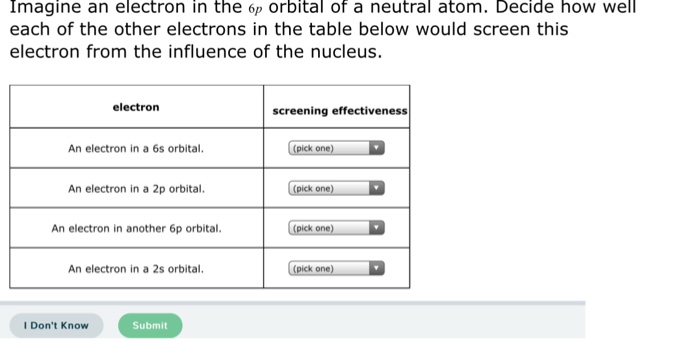
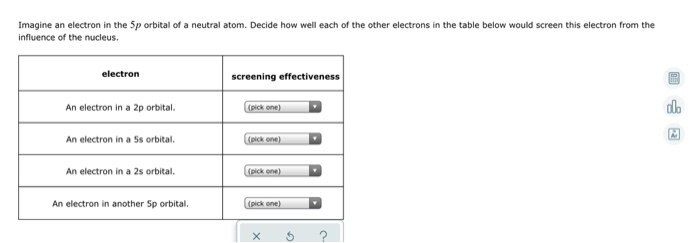
SOLVED: Imagine an electron in the 5d orbital of neutral atom Decide how well each of the other electrons in the table below would screen this electron from the influence of the
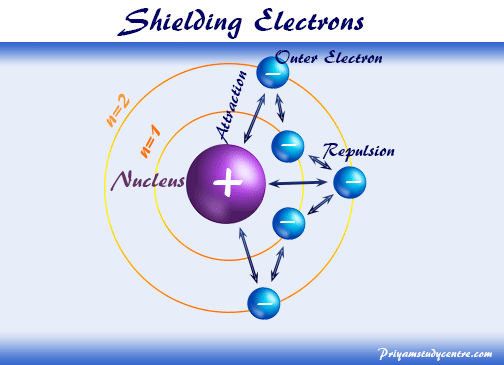
Welcome to Chem Zipper.com......: Effective Nuclear charge (Z* or Zeff): Slater's rule: Screening effect or Shielding effect

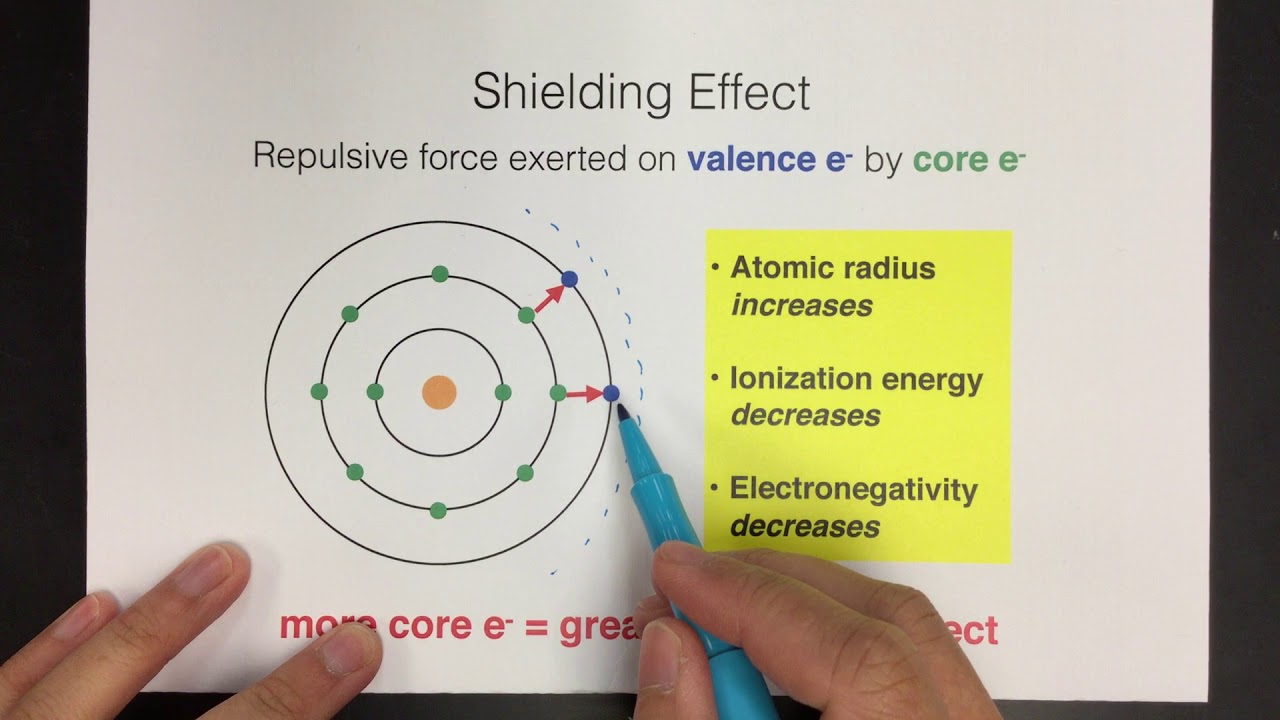
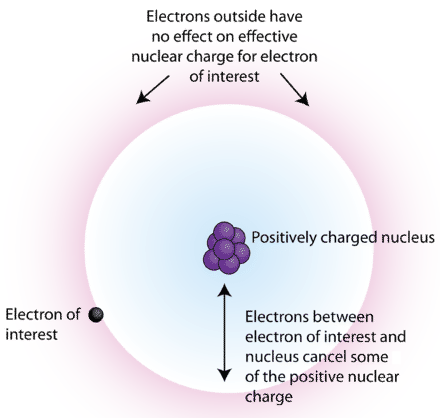
What is the Difference Between Effective Nuclear Charge and Shielding Effect | Compare the Difference Between Similar Terms

SOLVED: Question 37 (1 point) Screening of the nuclear charge by core electrons in atoms is: less efficient than by valence electrons more efficient than by valence electrons essentially identical to that